Quantum Enabled Drug Development
The race is on to build the world’s first usable quantum computer. The upside as a defense, artificial intelligence, and computational asset is undeniable, but how far away are we from ‘quantum supremacy’, the point where quantum computers are able to outperform classical computers? Specifically, how can we use this up and coming technology to make an impact on human health, specifically on new drug discovery and development?
Current Quantum computers
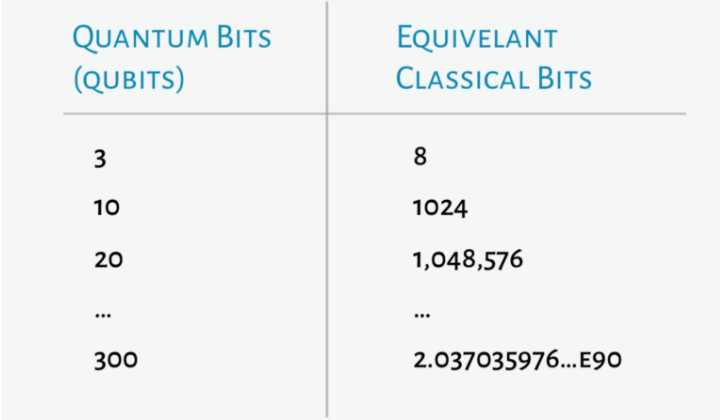
Quantum computing was first described in the early 1980s, but only recently with the attention of tech giants including Google and IBM, has it gained momentum as a potentially transformative technology. It relies upon quantum-mechanical phenomena such as tunneling, superposition, and entanglement to perform computation, with units of information transmitted in quantum bits (qubits) instead of traditional bits of ones and zeroes. Unlike traditional computing, qubits can be in a 0 state, a 1 state, or in between the two states, as a superposition of the 0 and 1 states. Quantum computing has an advantage over traditional computing when many factors or elements are considered due to efficiency in combinatorial optimization. Massive parallelism that can be achieved by modeling many solutions simultaneously. The number of solutions that can be modeled simultaneously doubles with each additional quantum bit, or qubit, added to the system, allowing exponential scaling far beyond anything achievable with classical computers for certain classes of search problems.
A Perfect Use Case for AI
The first low scale prototypes of quantum computing systems are available. However, it is still an immature technology with minimal usage in the short term due to high costs, high error rates, and low compute power. Limited molecular simulations have been done on simple structures such as a chain of hydrogen atoms by Google in late 2019, or simple 3 atom molecules like beryllium hydride (BH2) by IBM in 2017. As a comparison, the largest reported molecule simulated using classical computing was pentacene, 36 atoms total. The size of most small molecule compounds are between 50 and 80 atoms, with biologics and protein therapeutics orders of magnitude larger than that.
The state-of-the-art today includes quantum computers with 53 and 72 qubits in size available from IBM and Google respectively, with an expected doubling in the number of qubits every one to two years. Scientists are estimating that the penicillin molecule that would have required 1086 classical bits could be simulated with a quantum computer that has 286 qubits. If the current pace of development holds, quantum computers of that size should be available later this decade.
Quantum Computers in Drug Development
The application of quantum technologies towards drug discovery can be classified into two focus areas: quantum mechanics and quantum computing. Technologies utilizing quantum mechanics refer to the use of improved physical models to describe drug target interactions, resulting in accuracy improvements over traditional molecular mechanics models. Quantum computing is a new paradigm of computing that offers improved speed and efficiency for particular computational pain points including simulation of quantum mechanics and simulation of large molecules and biologics.
The biopharma industry already applies quantum mechanics for energy calculations and structural optimization, especially in molecular docking and quantitative structure-activity relationship (QSAR) analyses. Quantum mechanics–enabled synthetic chemistry gives researchers the tools to preclude potentially inactive compounds. But the usefulness of virtual tools depends on their ability to accurately predict hits, especially for complex molecules. Classical computing techniques to ‘brute force’ the calculations by simulating the atomic behavior of molecules is slow and inefficient. The industry has been trending towards the use of AI to learn how to most efficiently convert compute power into accurate insights, but these are still in development and have a ceiling to how accurate and fast they can be.
Quantum computing has the potential to transform virtual screening through physically precise modeling of drug-target interactions and efficient screening of massive virtual libraries. The qubit based computing architecture most closely emulates the probabilistic quantum mechanics models that are used to describe molecules, and the strengths of quantum computing including multiparameter optimization, linear algebra, and search make the technology an ideal engine for several use cases.
Commercial Value
Quantum aided drug development strategies are immature, and as a result, many companies have taken a hybrid approach, combining quantum inspired algorithms with classical computing architectures. These algorithms currently have limited use cases, and the majority of which have provided improvements over existing computational drug design strategies primarily the de novo design of protein therapeutics. The hardware and infrastructure allowing the scalable utilization of quantum computing is still >3 years away and currently has problems in both accuracy and compute power.
The industry leader in the startup space is XtalPi, a B stage company that has raised $67.7 million from investors including Sequoia China, Tencent, SIG China, Google, Morningside VC, and ZhenFund. The company was founded in 2014 by quantum physicists from MIT, and their CEO is Jian Ma. In 2018, XtalPi engaged in a research partnership with Pfizer to develop their Intelligent Digital Drug Discovery and Development (ID4) platform. With tightly interwoven quantum physics, artificial intelligence, and high-performance cloud computing algorithms, XtalPi’s ID4 platform provides more accurate predictions on the physiochemical and pharmaceutical properties of small-molecule candidates for drug design.
Pharmaceutical giants are in wait and see mode in regards to research collaborations with quantum computing startups. AstraZeneca, Biogen, Merck, GSK, and Pfizer (above) have engaged in exploratory stage research collaborations with emerging startups in recent years, but all have just gotten started (past 3 years). However, as large biopharma companies have increasingly searched for new ways to support R&D platforms with declining productivity, it is reasonable to expect more frequent future partnerships once the value proposition has been more fully demonstrated.
Evaluating Startups in the Space
The two largest challenges in the quantum drug discovery space are talent and infrastructure. Quantum computing is currently an exceedingly niche field that requires deep specialization in physics to understand hardware deployment and machine learning to develop effective algorithms and applications.
Current quantum computing offerings are early stage and expensive. The largest quantum computers are error prone and have not demonstrated supremacy over classical computing systems. Better suited to solve almost all drug discovery problems currently are domain expertise or the application of AI techniques on classical computing architectures. While current complete quantum computers have only reached a maximum size of 72 qubits, computers in the range of 300 qubits or more are necessary to outperform classical computers. Furthermore, the more qubits a computer has, the further the risk of calculation errors. Thus, there must be significant strides to improve both size and accuracy of quantum computers.
Another consequence of being an emerging technology is that talent is in very short supply. Mastery of quantum technology requires Ph.D. degrees in top academic institutions, in a quantum development environment. Quantum software developers have a very different skill set than ‘normal’ software engineers because quantum computing is at a far more basic stage of development which requires physical knowledge of the hardware and software interface.
Overall, quantum aided drug development is an immature field that requires intense domain expertise in the founding team as well as technology that does not rely on quantum computing hardware upgrades to provide value. The funding environment is favorable, and the startup cycle is young, with most startups at seed stages with less than $3 million in total funding. Technology that utilizes quantum chemistry to improve drug binding models can provide marked improvements over classical molecular mechanics models and have value as part of a screening process. However, these approaches seem better suited as part of an arsenal of tools led by a machine learning driven screening platform rather than as a standalone solution. Quantum computing approaches are years away and will take time to mature. Hybrid approaches applying ‘quantum inspired’ algorithms towards protein design and other approaches with heavy computational load are promising but still without an example of a scaled proof of concept.
The entire investment space is uncharted territory and thus, investments into this space need to be made only on strong teams with the ability to pivot or reapply their technology to an adjacent need. Still, the next decade will see drastic improvements in quantum computing speed and accuracy. Startups should validate proof of concepts and ensure that they are on the frontier of high performance computing in order to take advantage of future advancements.